CAMBRIDGE, Mass.--(BUSINESS WIRE)--
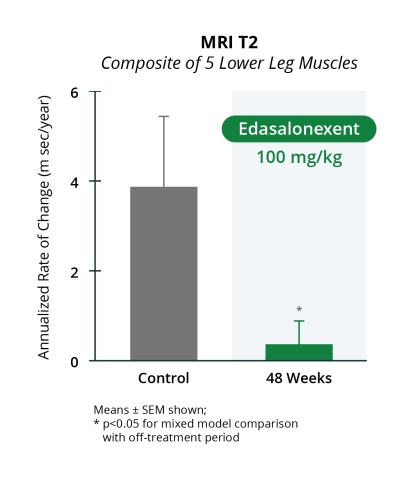
Catabasis Pharmaceuticals, Inc. (NASDAQ:CATB), a clinical-stage biopharmaceutical company, today reported new positive magnetic resonance efficacy results showing slowed disease progression in boys with Duchenne muscular dystrophy (DMD) in the MoveDMD trial through 48 weeks of edasalonexent treatment. Both magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) were performed in the Phase 2 MoveDMD trial and open-label extension. Statistically significant improvement in the rate of change in lower leg composite MRI T2 at 12, 24, 36 and 48 weeks on oral 100 mg/kg of edasalonexent treatment were observed compared to the off-treatment control period (p<0.05 for all time points). Improvements in changes in both soleus and vastus lateralis (VL) MRS fat fraction through 48 weeks of edasalonexent treatment compared to the off-treatment control period were also demonstrated. These data were presented today at the American Academy of Neurology 70th Annual meeting in Los Angeles, CA. These improvements in MRI T2 and MRS fat fraction show a slowing of disease progression and are in addition to the improvements observed in all assessments of muscle function through more than a year of edasalonexent treatment.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20180425006371/en/
(Graphic: Business Wire)
“The results reported to date from the MoveDMD trial have been consistent and support edasalonexent as a disease-modifying therapy. Overall, in the MoveDMD trial edasalonexent has slowed progression of this disease based on improvements in multiple assessments of physical function and biomarkers of muscle health and inflammation,” said Jill C. Milne, Ph.D., Chief Executive Officer of Catabasis. “We believe that these effects ultimately will translate to boys with Duchenne maintaining functional abilities longer.”
“I am very encouraged to see these emerging data with favorable MRI changes and results of muscle function assessments with edasalonexent, which are clearly divergent from the untreated disease progression that I see in boys in this age range with Duchenne,” said Richard Finkel, M.D., Chief, Division of Neurology, Department of Pediatrics at Nemours Children’s Hospital, Orlando and a Principal Investigator for the study. “There is a strong need for a therapy with a safety and tolerability profile like edasalonexent for the many boys affected by this devastating disease.”
MRI is a non-invasive approach to assess disease progression in DMD. MRI T2 as well as MRS fat fraction and T2 were performed in the Phase 2 MoveDMD trial and open-label extension. MRI T2 measures combined inflammation and fat in one measurement. MRS T2 measures the inflammatory component independently and MRS fat fraction measures the amount of fat in the muscle. As boys with DMD get older, the amount of fat in their muscles increases with consequent loss of functional abilities. Changes in MRI T2 and MRS fat fraction are known to correlate with changes in function in boys with DMD. Increases in both measures strongly correlate with worse performance on timed function tests and predict future loss of functional abilities. FDA considers MRI to be an important supportive early endpoint demonstrating therapeutic effect as mentioned in the recently released FDA guidance on development in DMD.
Statistically significant improvement in the rate of change in lower leg composite MRI T2 at 12, 24, 36 and 48 weeks of oral 100 mg/kg of edasalonexent treatment were observed compared to the off-treatment control period (p<0.05 at all time points). After 48 weeks of 100 mg/kg of edasalonexent treatment, MRS fat fraction of the soleus muscle increased by an average of 0.85%, while in the same boys during the off-treatment control period the average annualized increase was 2.6% per year. Boys from the ImagingDMD natural history study who were largely on steroids had an increase in soleus fat fraction of approximately 3% per year. After 48 weeks of edasalonexent treatment, MRS fat fraction of the VL (one of the quadriceps) increased by 5.9%, while in the same boys during the off-treatment control period the average annualized increase was 10.4% per year. Boys from the ImagingDMD natural history comparison study who were largely on chronic steroids had an increase in VL fat fraction of approximately 7% per year. MRS T2 of soleus and VL decreased from baseline. The changes in the rate of increase in fat fraction in the soleus and VL muscles demonstrate greater stability with edasalonexent treatment in the MoveDMD trial with less of an increase in fat in their muscles compared to the same boys prior to treatment. The increase in fat fraction observed on edasalonexent treatment was also less than that observed in natural history in a different population of boys with DMD.
Consistent with these magnetic resonance data, in the Phase 2 MoveDMD trial and open-label extension, a preservation of muscle function and slowing of DMD disease progression was seen in boys treated with edasalonexent compared to the rates of change during the control period prior to receiving edasalonexent. Through a year of treatment, the 100 mg/kg/day treatment group showed consistent and clinically meaningful improvements in rates of decline compared to rates of change during the control period across all four assessments of muscle function: the three timed function tests (10-meter walk/run, 4-stair climb and time to stand), as well as the North Star Ambulatory Assessment (NSAA), an integrated global assessment of muscle function.
Edasalonexent is being developed as a potential foundational disease-modifying therapy for all patients affected by DMD, regardless of their underlying mutation. No evidence of side effects or safety issues common with the current DMD standard of care has been observed after more than 37 patient-years of exposure to edasalonexent. Catabasis is preparing for a single global Phase 3 trial to evaluate the efficacy and safety of edasalonexent for registration purposes, dependent on raising capital.
About the MoveDMD Trial
The MoveDMD trial is
investigating the safety and efficacy of edasalonexent
in steroid-naïve boys enrolled at ages 4 – 7 affected with DMD (any
confirmed mutation). The trial is comprised of three parts - Phase 1,
Phase 2 and open-label extension. Phase 2 was a randomized,
double-blind, placebo-controlled 12-week portion with 31 ambulatory boys
across a range of dystrophin mutations. The 12-week MRI T2 primary
endpoint for treated boys compared to placebo was directionally positive
although not statistically significant. The open-label extension
evaluated longer term safety and efficacy using pre-specified analyses
comparing the rates of change in boys receiving edasalonexent treatment
and prior to treatment. In the Phase 2 and open-label extension of the
MoveDMD trial, edasalonexent substantially slowed DMD disease
progression in boys on 100 mg/kg/day up to 60 weeks of treatment. Across
all assessments of muscle function, consistent improvements were
observed in the rate of decline after 12, 24, 36, 48 and 60 weeks of
oral 100 mg/kg/day edasalonexent treatment compared to the rate of
change in the control period for boys prior to receiving edasalonexent
treatment. Statistically significant improvements were also seen in
non-effort based measures of muscle health (muscle enzymes and
c-reactive protein and MRI T2 compared to control). In the 100 mg/kg/day
treatment group, 16 boys commenced edasalonexent either at the beginning
of Phase 2 or at the beginning of the open-label extension.
Edasalonexent has been well tolerated with no safety signals observed in
the trial.
About Edasalonexent (CAT-1004)
Edasalonexent (CAT-1004) is
an investigational oral small molecule that is being developed as a
potential disease-modifying therapy for all patients affected by DMD,
regardless of their underlying mutation. Edasalonexent inhibits NF-kB, a
protein that is activated in DMD and drives inflammation and fibrosis,
muscle degeneration and suppresses muscle regeneration. Edasalonexent
continues to be dosed in the open-label extension of the MoveDMD Phase 2
clinical trial and Catabasis is preparing for a single global Phase 3
trial to evaluate the efficacy and safety of edasalonexent for
registration purposes, dependent on raising capital. The FDA has granted
orphan drug, fast track and rare pediatric disease designations and the
European Commission has granted orphan medicinal product designation to
edasalonexent for the treatment of DMD. For a summary of clinical
results reported to-date, please visit www.catabasis.com.
About Catabasis
At Catabasis Pharmaceuticals, our mission is
to bring hope and life-changing therapies to patients and their
families. Our SMART (Safely Metabolized And Rationally Targeted) Linker
drug discovery platform enables us to engineer molecules that
simultaneously modulate multiple targets in a disease. Our lead program
in development is edasalonexent for the treatment of Duchenne muscular
dystrophy. For more information on edasalonexent and our pipeline of
drug candidates, please visit www.catabasis.com.
Forward Looking Statements
Any statements in this press
release about future expectations, plans and prospects for the Company,
including statements about future clinical trial plans including, among
other things, statements about the Company’s plans to commence a single
global Phase 3 trial in DMD to evaluate the efficacy and safety of
edasalonexent for registration purposes, and other statements containing
the words “believes,” “anticipates,” “plans,” “expects,” “may” and
similar expressions, constitute forward-looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995. Actual
results may differ materially from those indicated by such
forward-looking statements as a result of various important factors,
including: uncertainties inherent in the initiation and completion of
preclinical studies and clinical trials and clinical development of the
Company’s product candidates; whether interim results from a clinical
trial will be predictive of the final results of the trial or the
results of future trials; expectations for regulatory approvals to
conduct trials or to market products; the Company’s ability to obtain
financing on acceptable terms and in a timely manner to fund the
Company’s planned Phase 3 trial of edasalonexent in DMD for registration
purposes; availability of funding sufficient for the Company’s
foreseeable and unforeseeable operating expenses and capital expenditure
requirements; other matters that could affect the availability or
commercial potential of the Company’s product candidates; and general
economic and market conditions and other factors discussed in the “Risk
Factors” section of the Company’s Annual Report on Form 10-K for the
year ended December 31, 2017, which is on file with the Securities and
Exchange Commission, and in other filings that the Company may make with
the Securities and Exchange Commission in the future. In addition, the
forward-looking statements included in this press release represent the
Company’s views as of the date of this press release. The Company
anticipates that subsequent events and developments will cause the
Company’s views to change. However, while the Company may elect to
update these forward-looking statements at some point in the future, the
Company specifically disclaims any obligation to do so. These
forward-looking statements should not be relied upon as representing the
Company’s views as of any date subsequent to the date of this release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20180425006371/en/



